
The evolution of earthworm nephridial symbionts
Symbiotic associations are widespread in nature and have been a major source of evolutionary innovation where symbionts have enabled hosts to conquer new niches by equipping them with novel functions. The transition from a free-living to a host-associated life-style has huge implications for the genome evolution of microbial symbionts. The textbook example is the extreme genome reduction in intracellular insect symbionts where the symbionts more resemble organelles than individual bacteria. Meanwhile, the genome evolution of extracellular symbionts is largely overlooked even though they are more widespread in nature. Marie Lund is studying the genome evolution of extracellular symbionts by using the nephridial (excretory organ) Verminephrobacter symbionts of earthworms as a model system.
The Verminephrobacter symbionts form a monophyletic cluster closely related to Acidovorax (betaproteobacterial), they are species specific and found in almost all lumbricid earthworms. The symbionts have co-evolved with their hosts, and the symbiosis likely originates in the last common ancestor of Lumbricid earthworms (62 – 136 million years ago). The symbionts increase the reproductive success of their host under nutrient poor conditions and are therefore considered beneficial. The vertical transmission takes place through the egg capsule (cocoon) where eggs, sperm, and symbionts are deposited after mating between the hermaphroditic worms. The symbionts can only colonize the developing embryo during a short window of opportunity early on in embryo development.
Genome sequences obtained from two cultivated Verminephrobacter symbionts, V. eiseniae and V. aporrectodeae, show no signs of genome reduction or AT-bias. However, genome comparison with closely related free-living organisms reveals slightly accelerated evolutionary rates in the symbionts and a high degree of shuffling of the gene order, both of which are among the first signs of drift-induced genome erosion. Marie Braad Lund wishes to investigate how genetic mixing, in the form of homologous recombination and horizontal gene transfer, lets the extracellular symbionts escape the deleterious effect of genetic drift.
Project title:
Faithful but not enslaved: Drives of genome evolution in microbial symbionts
Area of research:
Bioscience
Fellowship period:
1 Oct 2013 - 17 March 2016
Fellowship type:
Dale T. Mortensen Fellow

This fellowship has received funding from The Aarhus University Research Foundation.
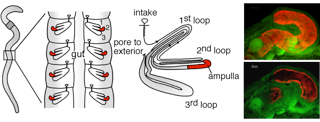
Figure:
The nephridia are found in pairs in each segment of the worm and consist of a long coiled tube leading from the opening to the coelomic cavity, through three major loops, finally exiting the body wall via an exterior pore. The Verminephrobacter symbionts reside in the ampulla where they form a dense biofilm lining the lumen wall. In red the Verminephrobacter symbionts are stained with fluorescence in situ hybridization (FISH) and the tissue auto-fluorescence is shown in green.