Acidic fish eyes see better
A recent study in eLife shows a new mechanism in the fish eye that boosts the retina´s oxygen supply more than 10-fold and enhances the eye's ability to process visual input. This mechanism for improved vision may have contributed to the extraordinary adaptive radiation of the fishes, which today represent half of all vertebrates in the world.
Visual input enters our eyes as light waves that are transformed by the eye´s light-absorbing retina into electrical signals that are send to the brain for interpretation. This energy-demanding process requires a lot of oxygen, which is supplied by a dense network of capillary beds in most animal tissues.
The fish retina does not possess an internal capillary network, likely to reduce light absorption by blood before the photons reach the photoreceptors. Therefore, oxygen´s diffusion distance is up to 50 times higher than in the human brain, which questions how oxygen can reach the retina without the typical capillary beds.
In the scientific journal eLife, researchers from Aarhus University together with international collaborators from Scripps Institution of Oceanography in the US and University of British Columbia in Canada, publish new results, where they identify a molecular mechanism within the fish eye that boosts vision by improving the oxygen diffusion through the retina by more than 10-fold.
A gas-gland within the fish eye
More than 300 million years ago, before the emergence of the first dinosaurs, fishes´ oxygen-binding protein, hemoglobin, mutated to become much more sensitive to acid. Here, acidification of the blood releases a large part of hemoglobin´s oxygen into the surrounding tissues. This includes the oxygen release into the swim bladder, so fishes can remain buoyant at great depths where extreme hydrostatic pressures prevail. However, the same mechanism for oxygen delivery may also allow fishes to deliver oxygen to their capillary-poor eyes.
“It has been known for half a century that the fish eye can generate high oxygen pressures within the eye to drive oxygen diffusion into their large eyes,” says Christian Damsgaard, AIAS Fellow and an assistant professor of animal physiology at Aarhus Institute of Advanced Studies and Department of Biology at Aarhus University, who is the lead-author of the new study. “However, the physiological mechanism that can generate these high oxygen pressures in the fish eye has remained highly elusive. This is what we have now identified.”
To identify the primary biochemical pathway involved in this superior mode of oxygen delivery in the fish eye, an international and interdisciplinary group of animal physiologists, molecular biologists, and pharmacologists joined forces.
The research group identified a set of enzymes that first pumps acidifying protons into the blood vessels that reach the eye. Then, the enzymes transport the protons into the red blood cells, where they shred oxygen off hemoglobin into the retina. These findings suggest that the vascular beds within the fish eye acts as an acidifying gas-gland similar to that found in their swim bladder.
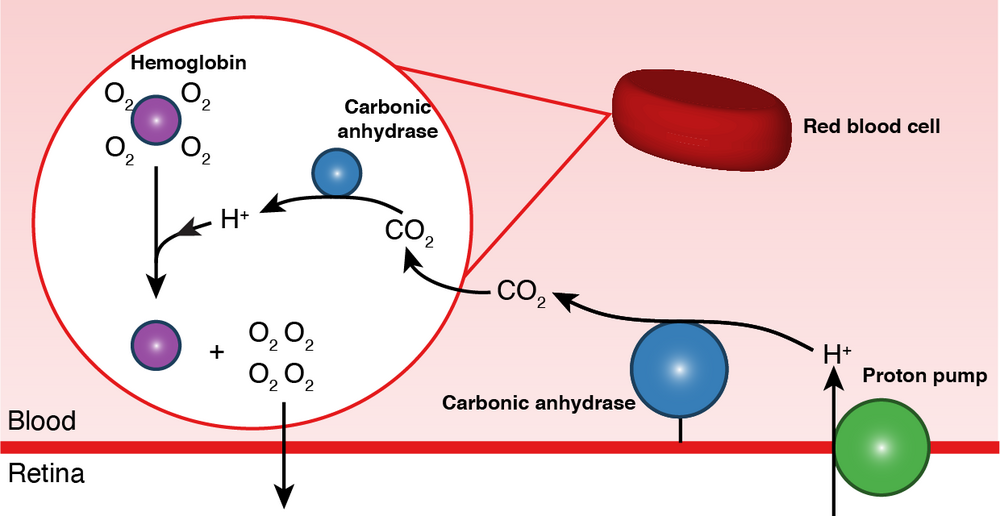
Image: Blood acidification mechanism for greatly enhanced oxygen supply to the fish eye. A proton pump (green) excretes protons (H+) into the retinal blood vessels lining the back of the photoreceptors. Carbonic anhydrase (blue) rapidly converts the protons into CO2 that diffuses into the red blood cell (red). An intracellular carbonic anhydrase converts CO2 back into protons that releases oxygen off hemoglobin (purple) into the retina. Modified from Damsgaard et al. 2020 eLife.
Oxygen boosts vision
To investigate how this newly discovered oxygen supply mechanism affects vision, the group measured the function of the retina while blocking the mechanism using pharmacological compounds.
Blocking the acidification mechanism in the fish eye rapidly impaired the function of the specific areas within the retina involved in signal-processing. Next, the group compared the anatomy of the retina in over 30 fish species and showed that species with the unique acidifying vasculature had markedly enlarged those specific signal-processing areas within the retina.
“These interesting findings strongly suggested that the evolutionary origin of this superior mode of oxygen delivery to the eye allowed fishes to better identify and track prey,” Christian Damsgaard explains. “This may have led to active feeding strategies in early fish evolution and fueled the adaptive radiation of fishes, which represent over half of all vertebrates,” he continues.
A proton pump with many functions
The same proton secreting protein involved in oxygen delivery in the fish eye is also found in many other aquatic organisms. They help sharks control blood pH, improve photosynthesis in algae within corals, and help bone-eating worms dissolve bones. This suggests that the genes encoding for this proton pumping protein is found in distantly related animal species.
“I currently investigate if the capillary beds behind the bird eye can acidify the blood to supply oxygen in the retina similar to fishes,” Christian says. “Birds have the sharpest vision among animals, but it remains unknown how oxygen and energy is supplied into this highly energy-demanding tissue. Here, proton pumps in are likely involved in the improved oxygen delivery to the eyes of birds, whose physiology closely resembles that of mammals, including humans. Therefore, I am very interested in determining if such a mechanism for improved oxygen supply can help humans with cardiovascular diseases in the future.”
The scientific paper behind the results
"A novel acidification mechanism for greatly enhanced oxygen supply to the fish retina" by Christian Damsgaard, Henrik Lauridsen, Till S. Harter, Garfield T. Kwan, Jesper S. Thomsen, Anette MD. Funder, Claudiu T. Supuran, Martin Tresguerres, Philip GD. Matthews, Colin J. Brauner in: eLife
Link: https://doi.org/10.7554/eLife.58995
Contact
Christian Damsgaard, PhD, AIAS-COFUND fellow & Assistant Professor
Aarhus Institute of Advanced Studies &
Section for Zoophysiology
Aarhus University
Denmark
Phone: +45 26 84 05 38
E-mail: christian.damsgaard@aias.au.dk
Twitter: @c_damsgaard